Abstract
Background
M-protein, a secreted antibody of malignant plasma cells, is a gold standard biomarker for monitoring the disease status in multiple myeloma (MM) patients (pts). The development of peripheral blood based ultrasensitive (MRD) methods of M protein detection is of high interest and importance. In 20% of MM pts, the M-protein consists of only the light chain (LC) of the immunoglobulin (Ig) molecule. For these LC-only MM pts, disease monitoring is challenging due to low levels of M-protein in serum. Urine protein electrophoresis (UPEP) and immunofixation electrophoresis (IFE), or serum free light chain (FLC) lack the sensitivity and/or specificity to track the M-protein in these pts, while bone marrow-based assays cannot be performed frequently due to their invasive nature. Thus, we evaluated the performance of EasyM, a mass spectrometry(MS)-based, non-invasive, sensitive assay for monitoring M-protein levels in LC-only patients in the MCRN-001 Canadian national and MD Anderson VRD-panobinostat frontline trials.
Methods
MCRN-001 trial is evaluating enhanced conditioning prior to ASCT for newly diagnosed MM (NDMM). After treatment with bortezomib (BTZ) based induction eligible MM pts received BuMel prior to ASCT. Busulfan was administered via IV at 3.2 mg/kg on days -5 to -3, or days -6 to -4 pre-ASCT (day 0) and melphalan was given at 140 mg/m 2 on day -2 or -3 pre-ASCT. Lenalidomide (LEN) administration began 100 days post-ASCT at 10 mg/d and continued until progressive disease (PD) onset. In the MDACC 2011-0192 frontline study in newly diagnosed MM, transplant-eligible pts received the novel combination of LEN, BTZ, dexamethasone (DEX) and panobinostat (RVD-panobinostat). The IMWG criteria were used to monitor clinical response in both trials. A total of 13 LC-only MM pts were selected for the study. Local IRB approval was obtained prior to the study. To derive the M-protein's full amino acid sequence, FLC was first enriched from the diagnostic serum sample. The FLC enrichment consisted of IgG depletion with protein A/G beads, followed by affinity purification of kappa or lambda LC containing Igs. Non-reducing PAGE was then used to separate FLC monomers, FLC dimers and full-length Igs. Finally, in-gel digestion of FLC monomers and dimers by multiple proteolytic enzymes were analyzed on a Q-Exactive mass spectrometer. Data analysis and sequence assembly were performed with the REmAb protein sequencing platform. To monitor M-protein levels in serum, unique, tryptic peptides from sequenced FLC were selected and quantified in diagnostic and follow-up samples with a PRM assay on a Q-Exactive instrument.
Results
M-protein sequencing
The full FLC sequence was derived for 8 out of 13 (61.5%) LC-only pts. The M-protein was successfully sequenced even when the FLC concentration was as low as 147 mg/L. However, FLC concentration measured by FreeLite assay was not a reliable predictor of our ability to derive the full sequence. The appearance of a sharp FLC monomer and dimer bands on PAGE after FLC enrichment was a better predictor of the M-protein sequencing success.
M-protein monitoring
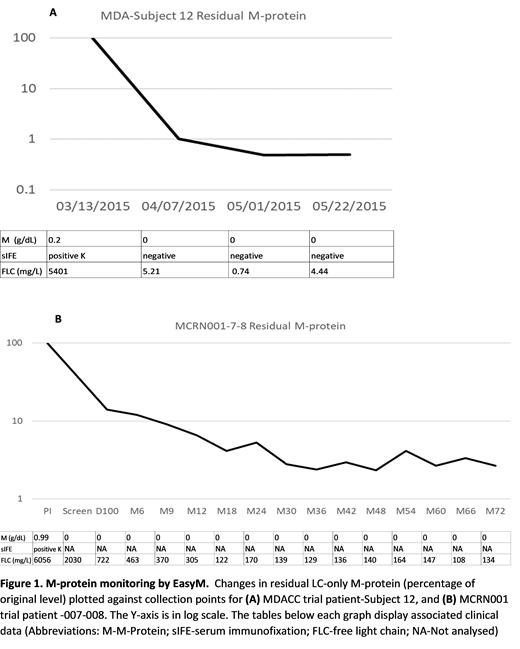
The M-protein of one pt did not contain any unique peptides. This pt was excluded for further analysis. In the remaining 7 pts, the M-protein contained at least one unique peptide and could thus be monitored by EasyM. For 6 LC-only pts, the M-protein monitored by EasyM correlated with the disease status measured by serum FLC, UPEP and urine IFE. A separate serial dilution test estimated that the limit of quantification can reach as low as 0.13 mg/L. Figure 1 shows representative data for two LC-only patients. One pt experienced relapse during the study; however, this relapse could not be detected by EasyM. This result could indicate a possible clonal switch at time of disease progression, but further investigation is needed to verify this.
Conclusions
Due to the rapid turnover and clearance of light chains conventional blood/urine tests have lacked the higher sensitivity needed to monitor disease state in LC only MM patients. To overcome the lower FLC concentration the current study successfully applied LC enrichment strategies to enable the sequencing and high sensitivity monitoring of FLC M-protein by MS in blood. The EasyM LC assay is non-invasive, sensitive, capable of assessing disease status, and has potential to be further investigated as a peripheral blood myeloma response and MRD monitoring biomarker.
Ma: Rapid Novor Inc.: Current holder of individual stocks in a privately-held company. Reece: Millennium: Research Funding; Amgen: Consultancy, Honoraria; GSK: Honoraria; Karyopharm: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; BMS: Honoraria, Research Funding. Manasanch: GSK, Secura Bio,Takeda, Celgene, Sanofi, Janssen and Adaptive Biotechnologies: Consultancy; Sanofi, Quest Diagnostics, Novartis, JW Pharma, Merck: Research Funding. Orlowski: Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria; CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees. Trudel: GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Roche: Consultancy; Genentech: Research Funding; Pfizer: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Sanofi: Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal